In new ton media equivalent attribute growth as pointed out in Desk-I need to be observed in indicative assets test.
By diversifying occupation progression choices, companies can improved match people' capabilities Using the obtainable opportunities and lower the potential risk of advertising men and women into sick-suited managerial positions.
It can be supplemented with supplemental parts or indicators to help distinct growth necessities or to detect certain metabolic things to do or reactions.
4. Does it ought to be performed every time the solution is tested or throughout the method validation or could it be feasible to get it done periodically?
Good Medium Acceptance Criteria – Common the number of colonies through the new batch of medium and the quantity of colonies from the Earlier accepted batch of medium. For the new batch of medium to get approved, the subsequent acceptance criteria must be fulfilled for every microorganism tested:
It should also be constant and Recurrent adequate to create a degree of basic safety involving the worker and their manager. On top of that, corporations need to prioritize standard overall performance evaluations and create mechanisms for upward feedback, enabling staff to deliver enter by themselves career development. This can be performed through surveys, feedback periods or conversations.
This is certainly verified by identification tests. The merchandise complies Together with the test if colonies of the kinds described will not be existing or if the confirmatory identification tests are destructive.
Use the microorganism strains suggested by the more info pharmacopeia. The strains should be not more than 5 passages from your reference tradition.
Sterility test atau uji sterilitas adalah suatu metode untuk mengetahui sedian farmasi atau alat kesehatany ang dipersyaratkan harus dalam keadaan steril. Dengan demikian sediaan dan peralatan tersebut harus bebas dari mikroorganisme.
Assess visually, the colonies figures on The brand new media agar plates While using the colonies figures to the Beforehand approved media agar plates as per annexure-two, Growth Endorsing, inhibitory and Indicative Homes of Media.
Moreover, this approach is most likely flawed in which the inoculum does not include a COA in addition to a gradual decrease in viability may not be easily detected. Testing with a reference substance presents an impartial and precise exterior calibration place.
For each day ready media GPT shall be executed as being a positive Command test with the respective microorganisms talked about in Table – one.
Cherwell also maintains the personal touch that accompanies a capability to support buyers’ one of a kind desires with bespoke answers. Cherwell’s new Growth Promotion Testing Tutorial is obtainable for down load here.
It click here notably concentrates on media employed for pharmaceutical and sterile professional medical unit market applications; including environmental monitoring; process and operator validation; and product or service sterility testing.
 Tia Carrere Then & Now!
Tia Carrere Then & Now!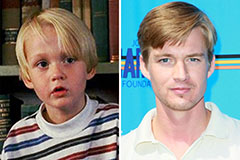 Mason Gamble Then & Now!
Mason Gamble Then & Now! Molly Ringwald Then & Now!
Molly Ringwald Then & Now! Destiny’s Child Then & Now!
Destiny’s Child Then & Now! Hailie Jade Scott Mathers Then & Now!
Hailie Jade Scott Mathers Then & Now!